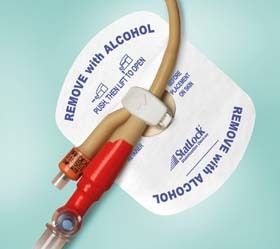
Statlock Foley Stabilization Device
This device is by far the best on the market. It makes wearing a catheter much easier and more confortable.
780-434-3131

STATLOCK Stabilization Devices are a more effective alternative to tape in helping improve clinical outcomes, quality of care and economic efficiencies.
The STATLOCK Foley stabilization device accommodates latex 8-22 Fr. and silicone 8-26 Fr. catheters for the ultimate in versatility.
INDICATIONS FOR USE: The STATLOCK stabilization device is compatible for medical tubes and catheters.
CONTRAINDICATIONS: Known tape or adhesive allergies. Known sensitivity to benzoin. (Zinc Oxide PICC Neonatal Universal)
WARNINGS AND PRECAUTIONS
25 Each per Box. Sold as single units
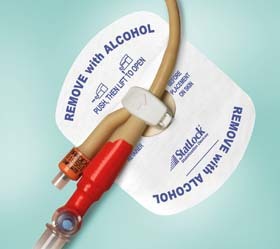
STATLOCK Stabilization Devices are a more effective alternative to tape in helping improve clinical outcomes, quality of care and economic efficiencies.
The STATLOCK Foley stabilization device accommodates latex 8-22 Fr. and silicone 8-26 Fr. catheters for the ultimate in versatility.
INDICATIONS FOR USE: The STATLOCK stabilization device is compatible for medical tubes and catheters.
CONTRAINDICATIONS: Known tape or adhesive allergies. Known sensitivity to benzoin. (Zinc Oxide PICC Neonatal Universal)
WARNINGS AND PRECAUTIONS
25 Each per Box. Sold as single units